
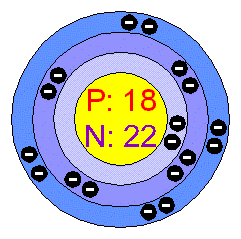
8 ways you can see Einstein's theory of relativity in real life Elementary, my dear: 8 elements you never heard of That means, it disappears much too quickly for a nucleus of magnesium-18 to even have the chance to cloak itself with electrons' and so it exists - and only very briefly - as "naked" nuclei. Most atomic nuclei quickly "cloak" themselves with electrons - particles with a negative charge - from their environment and become elemental atoms, which then can combine with atoms of other types to make chemical compounds.īut the newly-discovered magnesium-18 isotope is radically unstable and very short-lived: With so few neutrons, the nucleus quickly falls apart, with a half-life - the time it takes for half of its nuclei to disintegrate from radioactive decay - of less than one-sextillionth of a second, or 10^-21 seconds. One of the products of the resulting collision was the newly-discovered isotope, magnesium-18 - the "lightest" isotope of magnesium ever seen, with 12 protons and just six neutrons in its nucleus. Working against the clock, the researchers then fired the magnesium-20 nuclei - again at about half the speed of light - at yet another beryllium target, about 100 feet (30 meters) away. The collision in that step of the process yielded a "soup" of lighter magnesium isotopes the researchers could select from - among them the unstable isotope magnesium-20, which holds just eight neutrons per nucleus and radioactively decays in a few tenths of a second. They then fired the high-speed beam of magnesium nuclei at a target of metal foil made of beryllium. As a result, it's called magnesium-24.įor their experiments, the researchers accelerated a beam of magnesium-24 nuclei to about half the speed of light inside the National Superconducting Cyclotron Laboratory at MSU - a circular, ultra-high-energy particle accelerator. The most common stable isotope of magnesium has 12 neutrons - particles with a neutral charge - in each nucleus, giving this version of the element an atomic mass of 24.

Magnesium is relatively abundant in the Earth's crust and it has an important chemical role in many biological and industrial compounds. Like many chemical elements, magnesium originates in the fusion reactions of aging stars, and it's found on Earth because those long-dead stars have exploded as supernovas and "seeded" the interstellar clouds that formed our solar system.


 0 kommentar(er)
0 kommentar(er)
